Ashok Kumar Janakiraman
Muhammad Suhail 1 , Ashok Kumar Janakiraman 2* , Arshad Khan 1 , Abid Naeem 3 , Syed Faisal Badshah 1
1 Department of Pharmacy, Faculty of Pharmacy and Alternative Medicine, TheIslamia University of Bahawalpur, Punjab, Pakistan
2 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, UCSI University, Malaysia
3 Key Laboratory of Modern Preparation of Traditional Chinese Medicine, Ministry of Education, Jiangxi University of Traditional Chinese Medicine, Nanchang, China
Ashok Kumar Janakiraman, Department of Pharmaceutical Technology, Assistant Professor, Faculty of Pharmaceutical Sciences, UCSI University, Malaysia, Email: ashok@ucsiuniversity.edu.my
Janakiraman, A. K., et al. Surfactants and their Role in Pharmaceutical Product Development: an Overview (2019) J pharma pharmaceutics 6(2): 72-82.
© 2019 Janakiraman, A. K. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Surfactants are an extraordinary class of versatile amphiphilic compounds which have a spatially distinctive polar hydrophilic head and non-polar hydrophobic tail group. Due to its amphiphilic nature and unique feature of decreasing the interfacial tension, the surfactant is widely used in every walk of life such as individual care products, domestic cleaners, pharmaceuticals, oil recovery, food handling, and nanotechnologies. This review article will focus on the classification of surfactants, mechanism of action, antimicrobial functions, and especially emphasize the role of surfactants in pharmaceutical product development. Several, other practical application zones that are demonstrated in terms of foods and gene therapy, biological systems, health, and personal care products, petroleum and mineral processing are also briefly discussed.
Surfactants are surface-active compounds having the ability to decrease surface and interfacial tension at the interfaces between gases, liquids, and solids, thus enabling them to blend or diffuse voluntarily like emulsions in water or other liquids. The massive surfactant demand is currently fulfilled by several petroleum-based chemical surfactants. These compounds are non- biodegradable and usually toxic to the environment [1] .
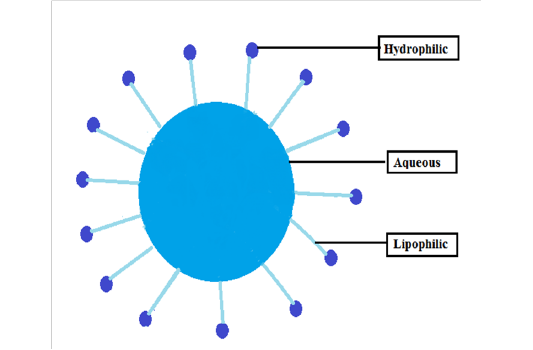
Surfactant molecules structurally consist of two portions. One portion is hydrophilic in nature and soluble in an aqueous medium, while the second portion is lipophilic, which is soluble in oil but insoluble in an aqueous medium. Structurally these two groups are contradictory in directions but their ends are linked to the same molecule, developing an asymmetric and polar structure. The structure is commonly expanded to “parent structure” (Amphiphilic structure) [2] . The molecules of surfactant possess amphiphilic arrangement providing an allurement for both water and oil as indicated in (Figure 1). Usually, the hydrophilic group possesses -SO 3 H, - COOH, and a polyoxyethylene chain; while lipophilic group normally possesses -CF 2 , -CF, - Si, and a polyoxypropylene chain. In surfactant molecules, the hydrophilicity and lipophilicity alter with molecular composition and arrangement. For example, when the lipophilic group is weaker than the hydrophilic, the surfactant becomes water-soluble and it becomes oil-soluble surfactant when a lipophilic group is stronger than hydrophilic part. Both water-soluble and oil- soluble yields a significant physio-chemical consideration of surfactant use. It is a significant source for a realistic surfactant’s choice [3] .
The surfactants play an active part in the designing and manufacturing of industrial and customer products, comprising cosmetics, detergents, paints, paper products, and pharmaceuticals [4] . Surfactants are employed too as emulsifiers throughout the conventional recovery of oil from deeply sited wells of oil [5] .
The last few decades displayed an enormous curiosity in work related to the synthesis of surfactants from natural products such as sugar-based surfactants fatty acids [6-8] and sterols [9] . These surfactants are fascinating as they usually are effortlessly biodegraded [10] . As compared to conventional surfactants, the demand of newly synthesized surfactants having additional properties is increasing at the industrial level. Moreover, a tendency to employ renewable natural products instead of toxic chemicals such as petrochemicals is also getting more attention.
The new period of global industrialization forces to change the traditional industries to adopt new developing technologies such as biotechnology, which possesses compelling advantages, and also provide many research opportunities. In 1980, the world market of the biotechnology was US $25 million which immensely grows to approximately US $1.7 billion in 1992 and is predicted to increase further than the US $500 billion through the century end [11] .
Mainly an inordinate contribution of industrial chemicals broadly employed in nearly all zone of modern industry is established by surfactants. During this period, the surfactant demand increased by approximately 300% within the chemical industry of the US [12] . Recent extensive production surpasses thirty lac tonnes annually (at a predictable price of US $4 billion) and is predicted to expand over forty lac tonnes through the end of the century [13,14] . Approximately, 54% of the total surfactants produced are employed in domestic / washing detergents, while 32% are only designed for industrial applications. Synthetic surfactants, mostly petroleum-derived, are the most abundant commercially available surfactants. Though, quick progress in biotechnology and improved ecological consciousness among customers, joint with the estimated different rule, has delivered additional stimulus for thoughtful deliberation of biological surfactants as promising substitutes to current yield [1] . The structure of a surfactant is shown in (Figure 1).

Figure 1: The structure of surfactant
Synthetic Surfactants and Biosurfactants
Surfactants are amphiphilic molecules having the domain of both hydrophilic and hydrophobic groups. The hydrophobic (non-polar) part is commonly a hydrocarbon chain and the polar part works in various modifications mode [15] . Ethylene, propylene oxide, sorbitan esters, ethoxylates, and copolymers are the most common non-ionic surfactants. Examples of commercially available ionic surfactants are sulphates (anionic) or ester sulphonates, quaternary ammonium salts (cationic) and fatty acids. Those microbial compounds that mostly show higher surface area and emulsifying activity are classified as biosurfactants. Biosurfactants are fundamentally different compounds which are prepared primarily through hydrocarbon-utilizing microorganisms revealing the activity of the surface. Biosurfactants may be formed using moderately modest and low-cost processes and substrates [16-18] .
By using biological systems, approximately different structural types of surfactants are produced, which cannot simply be produced by chemical processes. These particles may be tailor-made to garb different functions by altering the substrate’s growth or growth settings [19] . Biosurfactants are both biodegradable that is a positive biological feature [20-23] and non-toxic or less toxic as compared to chemical surfactants [24-27] . They ascend in soil certainly marks them adequate from a societal and biological point of view.
Classification of surfactants
Anionic surfactants: The anionic surfactants are liquefied in water producing the negatively charged surface-active group, whose aqueous solution is neutral or alkaline [28] . Based on the type of anionic surfactants, hydrophilic groups may be classified into five peptide condensates, namely: phosphate ester, fatty acid salt type, sulfate salt type, sulfonate, and carboxylic acid salt type. Anionic surfactants are the initial and most developed, and the leading class in several forms of surfactants. They may be broadly employed as foaming agents, antistatic agents, dispersants, detergents, emulsifiers, and stabilizers in the biochemical features of life [29] . The structure of anionic surfactant is shown in (Figure 2) [30] .
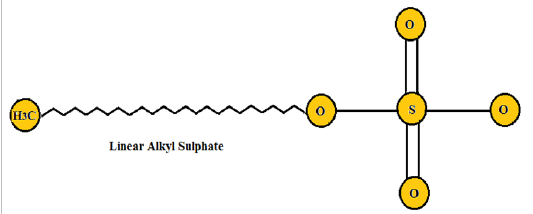
Figure 2: Linear alkyl sulphate (Anionic surfactant)
Cationic surfactants: Cationic surfactants are liquefied in water producing positive ions of the surface-active group. They possess great superficial action in an acidic medium and are expected to show swift and drop action in an alkaline medium. Based on chain arrangement, cationic surfactants are classified into open-chain cationic surfactants, heterocyclic group cationic surfactants and bonded intermediate connection cationic surfactants. Cationic surfactants are extensively employed for corrosion, rust, breaking, mineral flotation, and sterilization [31] . The generalized form of cationic surfactant is shown in (Figure 3) [32] .
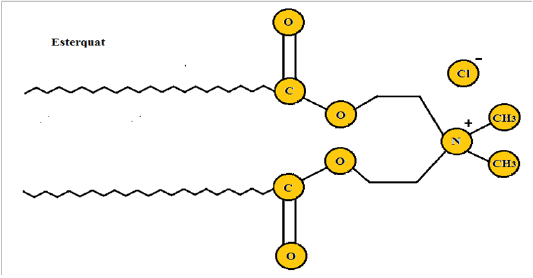
Figure 3: Esterquate cationic surfactant
Non-ionic surfactants: Nonionic surfactants are not ionized into any ions in an aqueous medium, and a high quantity of oxygen-containing groups form hydrophilic, accomplishing suspension through hydrogen bonding with water. Commonly non-ionic surfactants are existed both in liquid and slurry form, and their aqueous solubility reduces with temperature elevation. Non-ionic surfactants possess distinct physicochemical features from ionic surfactants due to their fundamental properties. Hydrophilic groups are classified into four types like polyhydric alcohols, polyethylene glycol type, glycosidic type, and polyether type. Non-ionic surfactants are broadly employed in the paper, textile, food, fiber, plastic, glass, medicines, dyes, pesticides, and many other productions. As compared to ionic surfactants, they show excellent performance; produced in higher amounts after anionic surfactants [33] . The general structure of non-ionic surfactant is shown in (Figure 4).
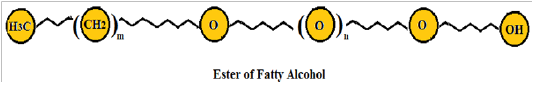
Figure 4: Ester of fatty alcohol (Non-ionic surfactant)
Amphoteric surfactants: Amphoteric surfactants possess both positive and negative ions. According to the anion type, amphoteric surfactants may be classified into lecithin, betaine, imidazoline, and amino acid- type. Amphoteric surfactants have minimal toxicity. It is moderate to the skin and possesses excellent biodegradability. Amphoteric surfactants have varied uses in the personal defending tools like shower gel, shampoo, cosmetics, etc. and also may be employed in industrial softeners and antistatic agents.
Speciality surfactants: Speciality surfactants possess various distinct features, which are lacking in conventional surfactants. The most important species is the fluorocarbon surfactant, which has high thermal stability, high chemical stability, and excellent surface activity in abundant varieties with a conventional surfactant exceptional part. Therefore, they are generally employed in the textile, fire protection, mineral processing, paper, pesticides, leather, and chemical industries. Moreover, Sn, Ti, and Ge elements are also employed to enhance the molecules of surfactant [34] .
Macromolecule surfactants: Commonly such surfactants raise to polymeric surfactants whose comparative molecular mass is higher than 10000, holding a surface-active element. Based on natural sources, they may be classified into natural, modified natural material and composing types [34] . Polymer surfactants may be applied as a gelling agent, thickener, emulsifier, fluidity-improving agent, antistatic agent, and dispersing agent. It has been known as a significant participant of the surfactants intimate.
Preparation of surfactants by a Fermentation process
Acylpolyols: Acylpolyols synthesized via fermentation are generally hydroxy fatty acids attached to disaccharides by ester bonds. They are extracellular compounds produced through actinomycetes like Mycobacterium, Corynebacterium, and Breibacterium [35] . The acyl polyols are present in higher quantity in bacterial cell walls.
Glycolipids: Glycolipids are commonly hydroxy fatty acids associated with sugar through a glycosidic bond. Sophorolipids and rhamnolipids are the clear examples produced through Candida and via Pseudomonas, correspondingly. Rhamnolipids are smart due to which they may be proficiently formed throughout development on both carbohydrates and hydrocarbon as the single source of carbon. There has been significant attention in rhamnolipid’s molecular genetics [36] .
A sucrose lipid formed through Serratia marcescens has recently been quarantined and characterized [37] . The yield was established to be an excellent emulsifier for an inclusive variety of hydrocarbons with crude oil. It has been also recommended as a surfactant to clean the oil tanks.
Acyl peptide: Acyl peptide (lipopeptides) is commonly known as cyclic compounds established on a short peptide chain and a hydroxy acid. Bacillus subtilis was used for the production of the most searched compound known as Surfactin [38,39] . Acyl peptide, as Lichenysin and Surfactin synthesized through Bacillus licheniformis, are proficient amphiphiles when it decreases the surface tension, etc. Surfactin is known as one of the rare biosurfactants which have established the commercial application. It is reprocessed in different ways to employ for pharmacological purposes. The only two biosurfactants which provide some controlling evidence are rhamnolipids and Surfactin, and their molecular genetics has also been studied.
Mechanism of Action of Surfactants
Surfactants can show its action in three different ways:
Roll-up procedure: The surfactant drops the oil/solution and fabric/solution interfacial tensions and boosts the fabric’s stain in this way.
Emulsification: The surfactant sinks the oil solution interfacial tension and prepares simple oil emulsification.
Solubilization: By interface with the surfactant micelles in a solvent (water), a constituent freely liquefies to make a constant and pure solution.
Surfactants are also widely known as wetting agents and foam formers. Surfactants are not only employed for making emulsion but also aids in eliminating fabric dirt / stains. Surfactants lower the surface tension of the medium in which it is melted. The surfactant plays a significant part in the entrapping oil phase through sinking the interfacial tension among interfaces or two media (such as stain / fabric, water / stain, air / water). The water lowers the surface tension and marks it simple to buzz oil that would be the source of washing dirt and lubricate dull dishes, garments, and other exteriors, and support to retain those oily dust or grease deferred in the water hence developing emulsions. The hydrophilic head or water-loving rests throughout the water, and it jerks the oil in the direction of the water.
Role of surfactants: Surfactants play a significant role in the preparation of different drug delivery systems. For the preparation of compounds which are not entirely soluble in an aqueous medium, pharmaceutically tolerable surfactants or co-solvents are classically used to enhance solubility. Polymeric micelles prepared through surfactants possess a complete set of unique features that mark them right favorable carriers of the drug for a wide variety of drugs. The poor solubility in biotic solutions exposed through approximately 50% of the drugs quiet rests the restriction in various routes of administration such as oral, transdermal, and parenteral. Amid the current approaches to overawed such problems, the encapsulation of hydrophobic drugs into polymeric micelles that consists of surfactants are unique smartest substitutes. A significant role is played by surfactant both in pharma and non-pharma field. Comprehensive research of surfactant inclusion and action in the medical field would disclose a wide variety of its potential in tonic usage. Tapering the research on each surfactant would assist in the medical science’s field on the way to an enhanced treatment for numerous complaints.
A surfactant such as pulmonary surfactant ensures significant implications beyond decreasing surface tension and modifying mechanical features which cause reduced animation of work. For example, the epithelium of lung is in continuous contact with the atmosphere and surfactant protects against infection via improving the pathogens’ elimination, modifying the inflammatory cell’s response, and raising the biophysical action of the lung. Hydrophilic proteins that play a minor role in the synthesis of surfactant has an excellent impact on antimicrobial activity. Though surfactant is a well-known RDS’ (respiratory distress syndrome) treatment in preterm infants, around has been no substantial clinical value for the usage of exogenous the surfactant in adult patients with ARDS (acute respiratory distress syndrome) so far. Advance studies are vital to be performed to search the surfactants’ opportunity by way of an immune-modulating therapy or planning small molecules which control the accessibility of surfactant constituents in respiratory disorders [40] .
Surfactants also play an essential role in controlling the the particle size of the polymeric nan- particulate system such as nanocapsules having penicillin-G in double emulsion synthesis; categorization and discharge of drug-loaded polybutyl adipate (PBA) nanocapsules with penicillin-G are discussed here. The nanocapsules were prepared by exhausting a technique such as double emulsion solvent evaporation, exhausting span, and tween as surfactants while dichloromethane as an organic solvent. Penicillin-G loaded nanocapsules with small quantities of both surfactants tend to possess higher burst discharge. The entrapment of penicillin-G may rise to 60%, and the release of the burst may also drop beneath 45% under optimum formulation conditions. In this situation, the point in which the nanocapsules possess merely 130 nm width will be significant [41] . The forces acting in micellar foam are shown in (Figure 5) [42] .
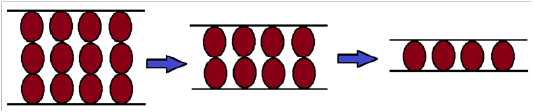
Figure 5: Structural forces in foam film (micelle)
Antimicrobial Function of Surfactants
Bacteria: Surfactant protein (SP-A and SP-D) are the hydrophilic proteins which play a dynamic role in the protection of host by stopping the growth of bacteria, simplifying uptake of bacteria through host cells, and accumulating and opsonizing pathogens [43] . These surfactant proteins may attach to both gram-positive and gram-negative bacteria. LPS resulting from K. pneumonia , E. coli , P. aeruginosa and Legionella pneumophila [44-48] may be interrelated by SP-A and / or SP-B, which subsequently results in adhesion, improving uptake of the pathogen, and inhibit growth. Furthermore, the surfactant proteins attach with peptidoglycan, a cell wall component of gram-positive bacteria derived from Staphylococcus aureus [49] and Streptococcus pneumonia [50,51] , as well as Mycobacterium avium, Mycobacterium tuberculosis, and Mycoplasma pneumonia to improve phagocytic uptake and stop their progress [52] .
Fungi: SP-A and SP-D can attach to a multiple of fungi, typically compliant pathogens, to simplify phagocytosis and agglutination the process through host cells. Animal researches establish that pulmonary collectins (SP-A and SP-D) improve the cell membrane permeability of H. capsulatum, preventing directly its progress [53] . They also bind to A.fumigatus, Blastomyces dermatitidis, Coccidioides posadasii, Cryptococcus neoformans and Pneumocystis jiroveci(carinii) [54-60] which causes in agglutination and increase awareness. Notably, such consequences seem to be specific to the microbe, by way of inter- relating of pulmonary collectins to Candida albicans inhibits phagocytosis through alveolar macrophages whereas quiet stopping the fungi growth [61,62] .
Virus: Pulmonary collectins (SP-A and SP-D) attach to viruses to expedite the elimination of the pathogen. Viruses are unique as compared to other numerous microorganisms in a way that they necessitate arrival into the cells of the host to reproduce. Because of SP-A and SP-Dexistences in the layer of mucus and surface of alveoli, they are in a good position to stop epithelial cells infection via agglutination, viral neutralization, and improved phagocytosis. SP-A and / or SP-D attach to hemagglutinin and neuraminidase of influenza A virus to stop their action [63] . Surprisingly, the pandemic influenza viruses’ hemagglutinin possesses a poor capability of attaching activity for surfactant protein D as associated with that of a seasonal influenza strain [64] . Pulmonary collectins also predicament to viruses’ glycoproteins, such as HIV RSV [65-67] and severe acute respiratory syndrome coronavirus [68] . Current studies specify that besides pulmonary collections, the surfactant lipid constituents also constrain the RSV infection [69] .
Application of Surfactants
Surfactants as enhancers for percutaneous immersion: The molecules’ permeation over the skin may be improved through the usage of assured adjuvant acknowledged as enhancers. By disarranging the layer of lipid of the stratum corneum and by keratin’s denaturation, ionic surfactants improve transdermal absorption. Enhancers may improve drug permeation as the stratum corneum swell and / or leak few of the structural components, therefore decreasing the diffusional conflict and improving the permeability of the skin.
One of the most excellent and well-organized enhancers of percutaneous absorption is azone. It significantly expands the synthesis of hydrophilic and hydrophobic compounds, the latter to a small grade. A promising action of azone is its fluidization of the intercellular lipid lamellar area of the stratum corneum. Azone is a precise nonpolar molecule that reaches the bilayers of the lipid and interrupts their structure. In divergence, dimethyl sulfoxide (DMSO) a powerful dipolar solvent, arrives at the aqueous area and interrelates with the lipid polar heads to procedure a huge solvation shell and increases the hydrophilic area amid the polar heads. Due to this, lipid fluidity is improved by both azone and DMSO, therefore decreasing the hurdle of the lipid barrier to the dugs’ diffusion. The permeability of drugs is also enhanced by alcohol derivatives of N, N disubstituted amino acids, and hexamethylene lauramine [70] .
Surfactants as flocculating mediators: To delay sedimentation of the floccules, a suspending agent is added regularly. These mediators are carboxymethyl cellulose, veegum, carbopol 934, tragacanth, or bentonites that are used both in single and combination state. It can cause incompatibilities, relying on the initial charge of the particle and the charge passed through the flocculating mediator and the suspending mediator. Through the incorporation of an anionic electrolyte like monobasic potassium phosphate, flocculating a positively charged particles are completed [71] .
Surfactants in mouth washes: The aqueous solutions, mouthwashes are frequently in the strenuous state comprising of one or more active constituents or excipients. They are employed via spinning the fluid in the oral cavity. Mainly for two purposes, mouthwashes may be employed such as cosmetic and therapeutic. Cosmetic mouthwashes can be synthesized to decrease lousy smell by the application of flavoring and / or antimicrobial agents. While therapeutic mouth rinses may be synthesized to decrease gingivitis, plaque, stomatitis, and dental caries. Surfactants are employed as they assist in the debris’ exclusion via delivering foaming action and in the flavors’ solubilization [72] .
Surfactants in respiratory distress therapy: To treat premature infants who are suffering from syndrome-like neonatal respiratory distress, which is also known as hyaline membrane disease, surfactant preparations are employed as a replacement therapy. In the US, around about 20% of the 250,000 premature born babies are suffering from such pulmonary disorder annually and accounts for 5000 deaths each year. A considerable shortage in the endogenous lung surfactant is the main contributing factor to the respiratory distress syndrome’s pathology. To assist the exchange of gas for either prophylactic or rescue treatment of neonatal respiratory misery condition, the preparations of lung, surfactants is employed in mixture with additional oxygen and motorized ventilation. The exogenous surfactants are either produced or derived from animals [73] .
Surfactants in suppository bases: Numerous nonionic surface-active agents, meticulously associated chemically to the polyethylene glycols, have been established as suppository vehicles 16. Several such bases may be employed for preparing both oil-soluble and water-soluble drugs. Sorbitan fatty acid esters (Span and Arlacel), polyoxyethylene stearates (Myrj), and the polyoxyethylene sorbitan fatty acid esters (tween) are most commonly used surfactants in suppository preparation. Carefulness might be implemented in surfactant usage with drugs. Many reports are representing enhanced absorption rate of drug, and other reports displaying communication of such surface-active agents with drugs and the resultant reduction in treatment action.
Surfactants in suspension aerosols: The surfactants and aerosol suspensions combination have been the highest prosperous. These surfactants use their action by coating all of the particles in suspension and turn at the solid-liquid interface. Agglomeration is condensed; thus, stability decreases if a physical barrier is provided. Rendering to the examinations approved through Young, Thiel, and Laursen [74] nonionic surfactants were considered to be the highest useful surfactants as related to other surfactants type. All such surfactants like sorbitan trioleate possessing an HLB less than 10 might be employed for dispersions of aerosol. Sorbitan sesqioleate, sorbitan monooleate, and sorbitan monolaurate are other agents that were found to be useful [75] .
Surfactants in water-based aerosols: Comparatively, high water quantities may be used to substitute completely or partly non- aqueous solvents used in aerosols. These yields are referred mostly as water-based aerosols and relying on the preparation they are released either by way of foam or spray. For the formulation of a spray, the preparation must comprise a dispersion of active constituents and added solvents in an emulsion system where the external phase is propellant. In such a manner, when the yield is distributed, the propellant evaporates and separates the active constituents into small particles. Meanwhile, water and propellant are immiscible, three-phase aerosol forms (water phase, propellent phase, and vapor phase).
To synthesize a suitable homogeneous dispersion, surfactants have been applied to a high level. Both low aqueous soluble surfactants and high soluble surfactants in nonpolar solvents have been found highly beneficial. Long-chain fatty acid esters of polyhydroxylic compounds containing glycerol, glycols, and sorbitol esters of stearic, oleic, palmitic, and lauric acid represent this sequence. Generally, surfactant approximately 0.5% to 2.0% is used. The content of propellent fluctuates from 25 to 60%, however, it may be as low as 5%, depending on the nature of the product.
Surfactants for contact lens cleaning purposes: As cleansers surfactants act, that emulsifies inorganic compounds, lipids and stored oils over contact lenses. Surfactant agents are exploited within an automatic device of washing and also through employing numerous drops of solution on the surface of the lens and moderately rubbing the lens backward and onward through thumb and forefinger or through placing the lens in the hand palm and rubbing gradually through a fingertip (approximately 20 to 30 seconds). The constituents in such cleansers typically contain a wetting agent, nonionic detergent, preservatives, and buffers [76] .
Surfactants in hard gelatin capsules: Aguiar et al . analyzed the dissolution of benzoic acid having low solubility presented as a free powder, and the same powder occupied into size 00 and 1 capsule respectively. The slowest rate of dissolution was found with the size 1 capsule where mostly the powder is firmly crammed. They stunned this problematic through the addition of polyol surfactant approximately 0.5% into the preparation. This highly enhanced the rate of dissolution that they presented was because of proliferation in the rate of disaggregation of the substance. Hydrophobic compounds have to be involved uncertainty in preparations due to necessities of filling machine, their harmful effect on the release of the drug may be overwhelmed through the accumulation of wetting agents, surfactants at 0.1-0.5% levels [77] .
Surfactants as emulsifying agents: The lipophilic protein of the molecule in a surfactant is commonly secretarial for molecule surface activity. Due to their different ionic charges, both cationic and anionic agents are likely to counteract each other if existing in a similar system and are therefore deliberated irreconcilable with each other. O / W emulsions are formed by few members of such group and others w / o emulsions depending upon their nature. Anionic emulsifiers contain several monovalent, polyvalent, and organic soaps like triethanolamine oleate and sulfonate like sodium lauryl sulfate, benzalkonium type of emulsifier. Nonionic type agents contain derivatives of polyoxyethylene and sorbitan esters. The surfactant’s ionic nature is a vital assurance for the selection of surfactants suitable for the formation of an emulsion. At pH range 3 to 10, nonionic surfactants are useful while at pH range 3 to 7, cationic surfactants show maximum effect, and anionic surfactants necessitate a pH which is higher than 8 [78-80] .
Surfactants as cerumen removing solutions: Cerumen is a permutation of the secretions of sweat and sebaceous glands of the external auditory canal. A sticky semisolid is formed by the secretions if permitted to dehydrate that grips ragged the epithelial cells, collapsed hair dust and further external particles that mark their approach into the ear canal. A casual gathering of cerumen in the ear can result in pain, itching and impaired hearing and is a restrictive to otologic checkup.
Over the centuries, hydrogen peroxide and light mineral oil have been usually employed mediators to relax obstructed cerumen for its exclusion. Lately, synthetic surfactant solutions have been established for their cerumenolytic action in ear wax elimination. Triethanolamine polypeptide oleate-condensate is one of these agents which is prepared commercially in propylene glycol, is employed for the cerumen emulsification, thus assisting its exclusion (Cerumenex drops). Additional commercial yield uses carbamide peroxide in glycerin/propylene glycol (Debrox drops). On interaction with the cerumen, oxygen is discharged by the carbamide peroxide that interrupts the reliability of the impacted wax, permitting its removal effortlessly [81] .
Surfactant inducing drug absorption: Surfactants affect the absorption of the drug from the gastrointestinal tract in humans. The membrane function and reliability can be interrupted hypothetically by the monomers of surfactant. Therefore, this disrupting effect of the membrane would incline to improve the penetration of drug and immersion through the gastrointestinal barrier. Drug absorption inhibition can arise as a significance of a drug being assimilated into surfactant micelles. If this type of micelles of the surfactant are not fascinated, which seems to be typically the case, and then solubilization of drug can cause a decrease in free drug absorption in solution in the gastrointestinal fluids that are accessible for fascination. Drug absorption inhibition in the existence of micellar surfactant concentrations would be predictable to arise in the case of drugs that are usually solvable in the gastrointestinal fluid, in surfactant absence. Still, in low soluble drugs condition whose absorption is dissolution rate limited, the enhancement in drug overload solubility through solubilization in micelles of surfactant could cause in the quick and fast dissolution rate of drug and therefore absorption. High surfactant quantities above which essential to solubilize the drug could reduce the absorption of the drug through reducing the chemical potential of the drug. Release of low soluble drugs from hard gelatin capsules and tablets can be improved through the surfactant insertion in their preparations [82,83] .
Surfactant in drug absorption from rectal suppositories: Riegalman and Crowell have presented that the rate at which the drug distributes to the suppository surface depends on the size of a particle of the suspended drug, and the existence of surface-active agents are the reasons which affect the release of drug from suppositories [84] . Surfactants can both rise and decline the absorption rate of the drug. For example, the absorption of sodium iodide is enhanced in surfactants presence and seems to be proportional to the relative surface tension dropping of the vehicle. Furthermore, Riegelman and Crowell identified that increase the absorption of sodium iodide might be too accredited to the peptizing mucus action of the vehicle. The rectal membrane is enclosed through a continuous blanket that can be extra freely splashed via colonic solutions which have decreased surface tension. The action of cleaning produced through the surfactant-containing vehicle can form other pore spaces accessible for drug absorption, therefore assisting the drug movement through the barrier of the rectal membrane. In phenol-type drugs case, the rate of absorption is reduced in the existence of surfactant, may be due to the creation of complex between a drug and surfactant.
Surfactants used in transdermal penetration of drugs: The penetration of drugs over the skin may be affected by surfactants. Sarpotdar and Zatz examined the lidocaine penetration over the hairless skin of mouse in vitro from vehicles comprising different combinations of polysorbate 20 and propylene glycol. The most appropriate and excellent solvent used for lidocaine is propylene glycol that decreases its segregating into the stratum corneum, dropping the penetration rate. In this research study, the effect of the surfactant is influenced by the concentration of propylene glycol in the vehicle. The flux reduction for 40% w / w concentration of propylene glycol can be clarified through solubilization of micellar of the lidocaine. It is typically presumed that the free drug has only the ability to penetrate the skin. Therefore the micellar solubilization of lidocaine decreases the vehicle thermodynamic activity and delays its penetration. At a maximum concentration of propylene glycol (60% and 80%), a rise in flux was detected, probably due to an interaction formed between surfactant and propylene glycol [85] .
Surfactants in microbiology: Surface active agents such as quaternary ammonium compounds are those compounds which have antibacterial activity in themselves. On the cell surface, the agents are adsorbed and bring about destruction by enhancing the leakiness or permeability of the cell membrane of the lipid. By damage of vital resources from the cell, death then occurs. Both gram-negative and gram- positive organisms are liable to the action of the cationic quaternary compounds, while gram- positive organisms are further condemned effortlessly through anionic agents as compared to gram-negative bacteria. As antibacterial agents, nonionic surfactants are slightly active and productive. They, frequently obstruct the growth of bacteria; apparently through giving a long fatty acids chain in a form which is digested easily through organism.
Ore floatation: On a liquid medium, the floatation of solid particles mainly relies on the angle of contact that may be altered through surfactant addition. The demonstration of acquainted fundamental chemistry comprises forming a float of the needle on the surface of water via coating it in wax. Continuous accretion of a household detergent drops the needle. The moralities are the equivalent as the crude mineral ores treatment through flotation, for that a minor quantity of collector oil is added throughout the slurring and grinding phenomena. The collector oil, which is an anionic, cationic, or non-ionic surfactant, turns to modify the ore particles wettability. Thiophosphates and organic xanthates and are mostly employed for long-chain fatty acids for oxide and carbonate ores and sulfide ores. In exercise, a foaming agent has commonly added also hence when air is passed over the suspension the ore particles unit to the bubbles of air. After that the particles of ore float to the surface whereby the process of skimming, they are recovered. This type of floatation is too employed as a purification technique for sewage and sludges [86] .
Surfactants and detergency: Detergency is the action of the surfactants, which causes assistances in the elimination of foreign particle from the surfaces of solid via adsorbing at interfaces and decreasing the energy required to effect the exclusion. Generally, most effective wetting agents are those who quickly diffuse and adsorb at suitable interfaces. Soap, a surface-active fatty acid salt comprising a minimum of eight carbon atoms [87] has been applied as a detergent for an extended time. Generally, soap has been acquired through the saponification of glyceride oils and fats with NaOH or KOH, yielding glycerol as a byproduct. The outstanding detergents in nature are soaps; however agonize from their sensitivity to acid pH and the existence of water hardness (Ca 2+ and Mg 2+ ions), producing soap foam. Though the detergent usage designers will recompense for such shortage, soaps have been fundamentally substituted through the commercial detergents. The bulk of manufacturing surfactant is enthusiastic to those constituents which are intermingled into the preparation of synthetic detergent.
Drag reduction: This sensation is fundamentally related to the multifold skill. Solutions of drag-reducing additives are typically visco elastic fluids possessing complex rheological features. Determining the features of drag-reduced turbulent flows demands for exclusively premeditated experimental and arithmetical model procedures and extravagant hypothetical deliberations. Appropriately accepting the mechanism of turbulent drag reduction needs grasping the turbulence basics and developing an appropriate connection between turbulence and the rheological properties persuaded through additives. Stimulating the drag reduction phenomenon applications necessitates the information from diverse areas like municipal engineering, mechanical engineering, chemical engineering, and so on. This book offers a detailed explanation of the turbulence features and theories, rheological behaviors, special procedures and application issues for drag-reducing flows via surfactant additives assembled on the state- of-the-art of scientific study outcomes by theoretical evaluates, numerical simulations and recent experimental studies [88] .
Gene therapy: The idea of gene therapy is to substitute unreliable or misplaced genes with well DNA carried into the cells through a carrier, known as a vector, in a transfection process. Commonly a positive charge is present on vectors that permit the DNA (negatively-charged) to pass over the hydrophobic membranes. Though, forecasting how effective a vector will be has been problematic. Verrall set out to explore how this might be enhanced. The set examined the interactions produced between DNA and synthetic vectors are known as Gemini surfactants. Gemini surfactants are lipids with two hydrocarbons, each linked to an ionic group, chemically associated through a spacer. These doubled-up surfactants have extraordinary transfection productivities; every molecule of lipid has double the positive charge of a single surfactant, permitting attaching to DNA at minor concentrations. It was found by Verrall’s group that DNA is filled firmly through the surfactants and that the surfactant-DNA complexes form on the arrangement of surfactant. They also indicated that there is a relationship between the efficacy of gemini surfactant’s transfection and a complex’s arrangement [89] .
Surfactants in nanotechnology: Having amphiphilic character permits self-assemble molecules of surfactant into variations of nanostructures from spherical/rod likes micelles to lamellar sheets. It is promising to plan a platform of surfactant proficient of solubilizing a higher quantity of oil whereas antagonistically water moistening the covering via using surfactant nanotechnology. By exhausting mixtures of surfactant, it is easy to make in-situ o/w microemulsions, permitting surfaces to be water damp. It may permit the removal of contaminated solvents [90] .
Analogous processes in biological membranes: Information on the solubility of antioxidant in reverse interfaces can assist in informing and recognizing the equivalent procedures in biological membranes. The reverse micelles are made, such as when a surfactant (Sodium bis (2-ethylhexyl) sulfosuccinate known as AOT) that can produce the reversed micelles in non-polar solvents [91] . The AOT molecules capability for aggregates outcomes from the mutual act of spatially disconnected hydrophilic and hydrophobic moieties of such surfactant. These aggregates are made as like interior cores filled through the hydrophilic head-groups coated through the hydrocarbon ends that are prolonged into non-polar solvent bulk. It has been exposed that the unusual the ability of AOT reversed micelles for a considerable quantity of water relies on the temperature and adjacent nonpolar medium (solute) and [92] .
Geometry parameters of micelles: Dynamic Light Scattering will employ to discover the geometry factors as the hydrodynamic the radius of the micelles as well as the same evidence about their movement by diffusion coefficient. Dynamic Light Scattering (DLS) acts through evaluating the intensity of light disseminated through the sample molecules as a function of time. Once the light is dispersed through a particle or molecule, few of the incidents light is disseminated. The quantity of scattered light would be persistent in case if the molecule were in a stationary position. Though, meanwhile, all solution molecules diffuse with Brownian motion in relative to the indicator, there will be intervention (constructive or destructive) that results in a modification in light intensity. By calculating the time scale of intensity of the light variabilities, DLS may deliver data concerning the size distribution, average size, and polydispersity of molecules and particles in solution.
Surfactants are surface-active compounds possess the capability of decreasing surface and interfacial tension at the interfaces between gases, liquids, and solids and show a vital role in the establishment and development of different pharmaceutical products by acting as dispersants, detergents, foaming agents, wetting agents and emulsifiers. They have many applications, such as eliminating dust from clothes, skin, and household objects mainly in kitchens and bathrooms, they are added. They are highly applied at the industry level. Cationic surfactants are extensively employed for breaking, corrosion, rust, mineral floatation and sterilization. The usage of non-ionic surfactants is extensively employed in the dyes, textile, pesticides, paper, fiber, food, glass, plastic, medicines, and other industries. Similarly, amphoteric surfactants have diverse uses in the individual protecting tools like cosmetics, shower gel, shampoo, etc. and may also be applied in antistatic agents and industrial softeners. Besides this, surfactants also perform antimicrobial functions as they stop the nourishment of various pathogenic microbes such like bacteria, fungi, algae, and virus, etc. and make the pharmaceutical preparations free of harmful microbes.